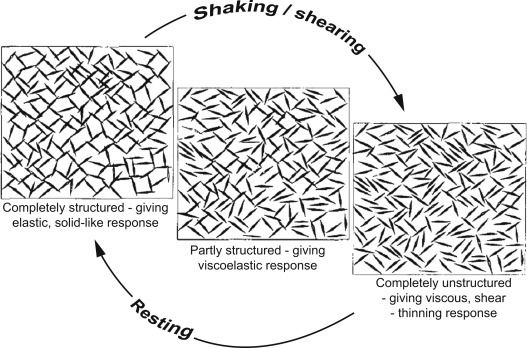
틱소트로피(thixotropy)
외력에 의한 고분자 물질 용액의 점성도(粘性度) 변화현상. 요변(搖變)이라고도 한다.
온도의 변화가 없는 상태에서 젓거나 흔들어주는 외부의 기계적 자극에 의해 겔(gel)이 졸(sol)로 되고, 이것을 방치해 두면 다시 겔로 되돌아가는 성질을 말한다.
수산화철 · 수산화알루미늄 등의 졸에 적당량의 염화나트륨을 가한 것, 펜트나이트 등의 서스펜션(현탁액), 안료 입자를 중합 아마인유에 분산시킨 페인트, 알루미늄비누를 윤활유에 분산시킨 그리스 등에서 볼 수 있으며, 마요네즈 · 토마토케첩도 틱소트로피를 나타내는 예이다.
일반적으로 입자의 형태 · 크기, 표면처리의 상태, 분산매의 종류 등에 의해 지배되는데, 콜로이드 입자가 비등방성을 갖고 입자 사이의 결합력으로 서로 허술하게 결합해 겔을 형성하기 쉬운 계(系)에서 나타난다.
이런 종류의 분산계에서는, 유동속도나 전단응력(剪斷應力)의 증가와 함께 유동저항(겉보기저항)이 감소하고, 전단응력이 감소하면 점성은 회복된다.
7.4.3 Thixotropy
https://www.sciencedirect.com/topics/chemistry/thixotropy
Thixotropy is a phenomenon by which the structure of a fluid is broken down under shear and rebuilt at rest (Barnes, 1997; Roussel, 2006b; Roussel et al., 2012). Thixotropy is therefore a reversible process, and a schematic representation of this can be seen in Figure 7.6.
Figure 7.6. Breakdown and build-up of a 3D thixotropic structure.
Reproduced from Barnes (1997) with authorization.5.08.2 Origins of Thixotropy
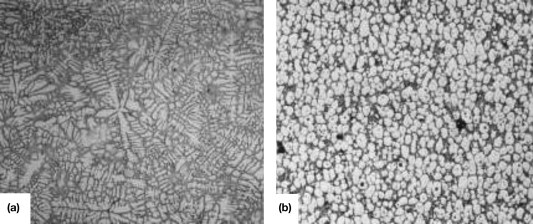
‘Thixotropy’ is shear-thinning property; when an alloy is sheared it thins, but when it is allowed to stand it thickens again. This behavior is associated with the breakup of agglomerates of solid particles under shear and their reformation when the shear is removed. The behavior is not observed when the microstructure is dendritic (as would normally be the case on solidification) but only when the microstructure in the semisolid state consists of spheroids of solid in a liquid matrix (Figure 1).
Figure 1. Micrograph of a typical (a) dendritic microstructure in an as-cast sample; and (b) a globular microstructure in a semisolid alloy sample.
At rest, these spheroids (or globules, as they are sometimes called) tend to form minute ‘welds’ between the particles. When the material is sheared, the bonds between spheroids tend to break (2). If the material is sheared for a long period of time at a particular shear rate, it reaches an equilibrium state characterized by a certain viscosity. This is associated with a characteristic agglomerate size, and the behavior is termed a ‘steady state.’ A change in the shear rate will lead to a tending toward a new characteristic viscosity, which is associated with a new characteristic agglomerate size (Figure 2).
Figure 2. Response of an inelastic thixotropic material to first a step change up in shear rate and then a step change down.
After Barnes, H. A.; Hutton, J. F.; Walters, K. An Introduction to Rheology; Elsevier: Amsterdam, The Netherlands, 1989.This is represented in a series of ‘flow curves’ in a diagram of shear stress against shear rate (3) (Figure 3). In this figure, point a corresponds to a shear rate γ˙1. At that point, the microstructure consists of a series of large flocs. If the shear rate is increased from γ˙1 to γ˙2, the flocs break up until the size corresponds to the flow curve that passes through point b. If the shear rate is then reduced back to γ˙1, the individual particles begin to collide with each other and agglomerate until an equilibrium size is reached, which is characteristic of the lower shear rate.
Figure 3. Flow curves of a flocculated suspension.
After Barnes, H. A. Thixotropy – A Review. J. Non-Newtonian Fluid Mech. 1997, 70, 1–33.When a step increase in shear rate is imposed, as in Figure 2, the shear stress will peak and then gradually decrease until it reaches the equilibrium value for the shear rate over time. The higher the shear rate after the step, the lower the equilibrium viscosity. Increasing the rest time before the step (if it occurs from rest) will lead to a higher peak viscosity before the viscosity recovers to the equilibrium viscosity for the shear rate specified.
Many materials systems show thixotropic behavior, but the origins of that behavior vary. So, for example, thixotropy in clay slurries is due to electrostatic attraction between unlike charges on different parts of the particle. In chocolate, it is based on the interlocking of growing crystals and their subsequent breakup. In food sauces, it can be traced to the entanglement of long molecules, and in mousses to the flocculation of bubbles. All these contrast with the mechanism in semisolid metallic alloys, where the formation of the minute welds is very similar to the processes involved in sintering. As the fluid is sheared, particles are forced into contact with each other. If it is energetically favorable for a solid–solid boundary to be formed (and this depends partly on the relative crystallographic orientation of the particles if they are single crystals), the two particles will stay in contact. If not, they will separate again. The process will be influenced by the rate of shear. So if the shear rate is hight the possibility of particle–particle contact is increased, but the time of contact is decreased. The formation of a new solid–solid boundary is time dependent. When the slurry is at rest, gravity will bring the particles into contact, and, gradually, a solid skeleton can develop. Once shearing is occurring, structural breakdown results; this will depend on the cross-sectional area of the bond and the radius of the neck, which generates a stress-concentrating effect.
'실험노트 > Nanoparticle ink stability' 카테고리의 다른 글
| Thixotropy, 예시와 함께 (0) | 2022.03.07 |
|---|---|
| Rheology 101 – Learning the Basics (0) | 2022.03.07 |
| 점성 (0) | 2022.01.17 |
| Slot die coating (슬롯다이 코팅) 조업 변수와 적용 점도 범위 (0) | 2022.01.14 |
| 코팅 방법(coating method) (0) | 2022.01.14 |